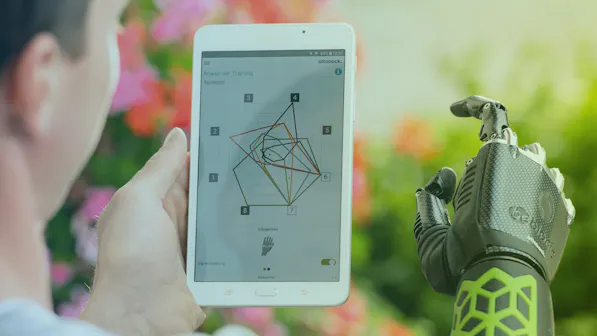
Software for medical devices
Special know-how
ISO 13485 - Quality management system
Software development according to IEC 62304
Requirements Engineering
Documentation
Risk management
Security, data protection
Device update capability
Automated processes CI/ CD
Device update capability
Medical device development since 2005
We are certified to ISO 13485 and develop software for medical devices in accordance with international standards such as the IEC 62304 software lifecycle process and the IEC 14971 risk management standard. We support you with risk management, documentation and software development and ensure traceability throughout the entire software lifecycle - a prerequisite for the approval of your product.
Thanks to our many years of experience in working with market leaders in the field of medical technology, we are very familiar with these requirements and deliver the appropriate solutions. For you, this means that we can seamlessly integrate our software into your processes as the manufacturer and "marketer" of the medical technology product.
Medical devices and their development phases are often highly complex. Most developments involve special know-how and high investments. We actively contribute to simplifying the complexities in the conception and implementation phase. Be it through the use of proven and sensible technologies to make the product robust in daily use or through optimized processes for correct documentation under medical law (e.g. according to software life cycle IEC 62304).
Read all the details about our specialist know-how below.
Selected customer projects
Your contact person